Remediation and Curation of the University of California , Riverside Collection of Aphytis
(Hymenoptera: Chalcidoidea: Aphelinidae)
J. Heraty, J. Pinto and S. Triapitsyn , PIs
Funding from the NSF is gratefully acknowledged
Remounting Procedures for Aphytis
Compiled by S. V. Triapitsyn in 1999
(at the initial stage of the project)
PROJECT SUMMARY:
Species of Aphytis are among the most important parasitoids of armored scale insects (Homoptera: Diaspididae). Many of the species used in classical and augmentative biological control programs around the world, including type and important voucher material, are represented in collections of the
The UCR collection of Aphytis formed the basis for a world taxonomic revision of the genus by David Rosen and Paul De Bach in 1979. The monograph was based almost entirely on the UCR collection of Aphytis, and because of this, it is an invaluable resource for basic and applied scientists. The UCR collection of Aphytis is almost entirely comprised of reared and slide-mounted specimens collected from 74 different countries and territories over the last 70 years. The magnitude and scope of the collection make it irreplaceable. Its revitalization will stabilize the existing collection and hopefully stimulate additional taxonomic studies of this important genus.
Because of their minute size, these wasps are generally ignored by collectors and are not accumulated in most insect collections. The UCR collection includes ca. 30,000 specimens of Aphytis mounted on ca. 5,000 slides. The majority of these specimens (28,224) are mounted in a temporary, water-soluble medium (Hoyer's) on 4,380 slides. A significant number of these mounts are now in various stages of decay. Many specimens, including numerous type specimens, have already been damaged. The purpose of this project is to protect the collection by transferring a significant portion to Canada balsam, a permanent medium better suited for preserving specimens in taxonomically significant collections of micro-Hymenoptera. Specimens are mounted individually and properly labeled. Currently, the majority of the material is mounted with an average of 6.67 specimens per slide, sometimes with more than one species per slide, and labeled very poorly.
REMOUNTING PROCEDURE
This procedure generally follows the one outlined by Platner et. al. (1999,) with a few modifications that are better suited for remounting Aphytis specimens. It allows specimens originally mounted in Hoyer's medium to be transferred to Canada balsam with minimal damage. A significant problem in remounting is that the antennae may collapse after contacting with the balsam. The following method prevents major collapsing in about 90% of the specimens. The rate of success in remounting and avoiding possible problems is the initial quality of the specimens: the better their original condition is, the fewer problems are encountered during the process of remounting.
The standard remounting procedure is as follows.
- Coverslips of Hoyer's mounts almost always have been sealed with Zut® or other compounds to reduce desiccation. This material is removed with the tip of a razor blade before processing.
- Slide is placed in a Petri dish and soaked in distilled water for 60-72 hours. After soaking, the coverslip can be lifted free of the specimen(s).
- Specimen(s) is/are transferred with a hooked probe to a ceramic depression plate supplied with 10% ethanol, then covered with a 6mm coverslip.
- Ethanol is replaced with 10% KOH for 30-40 min. (if the specimen is already clear enough) or up to 24 hours (if the specimen has not been cleared in the original slide). At this point, the quality of the original specimen can be improved somewhat. KOH not only is a clearing and softening agent but also reduces head and antennal collapse. Therefore, specimens must be treated with KOH even if they had previously been cleared in the Hoyer's medium. Because of flattening from the previous mount it is very difficult to reposition specimens during the remounting process.
- KOH is removed with a pipette and replaced with 10% ethanol for 30 min. The dehydration procedure is repeated with 20%, 40%, 60%, 80%, 95%, and twice with 100% ethanol.
- Absolute ethanol is replaced with a 1:1 mixture of 100% ethanol and clove oil. The depression plate with specimens is placed into a partly opened container for 2-3 weeks to allow for slow, complete evaporation of the alcohol and any remaining water. Once evaporation is complete only clove oil will remain and the specimen is ready for mounting.
- Using the balsam applicator, a small drop of Canada balsam (pre-mixed with 15% clove oil) is picked and placed in the center of a clean slide, which sits in a template to assist proper positioning of the specimen at the center of the slide. A dot of mountant ca. 3-4 mm in diameter is optimum, but this dot should not be mixed or spread significantly. The specimen is gently placed in the mountant by removing it from the clove oil with a hooked probe and submerging it, dorsum up, in the balsam drop. At this point the specimen can be oriented and some minor repositioning of body parts is possible. It is important to do it quickly, not allowing the mountant to dry after specimen placement. Dry 6 mm coverslips (precleaned with 80% ethanol and lens tissue) is then immediately placed flat, parallel with the slide, on the specimen. This allows it to contact the balsam drop near its center and forces air bubbles out when pressure is applied with the forceps.
- The slide is marked with the individual code to assure proper labeling, and a corresponding male (M) or female (V) symbols is marked on the slide in such a way that they indicate positioning of the specimen on the slide. The slide is then labeled in such a way that specimen's head is positioned upside down relative to the label; this is necessary to assure that when this specimen is viewed using a compound microscope, it appears in the right position with its head up.
- The slide(s) is/are placed onto slide warmer (50°C) for 3-7 days.
LABELING
The original slide (usually without specimens) is kept for historical purposes and is the main source of labeling information. However, because many slides do not contain proper labeling data, when possible we compliment the original data with complimentary information available either from Rosen & De Bach (1979), other relevant publications, or from the UCR quarantine records.
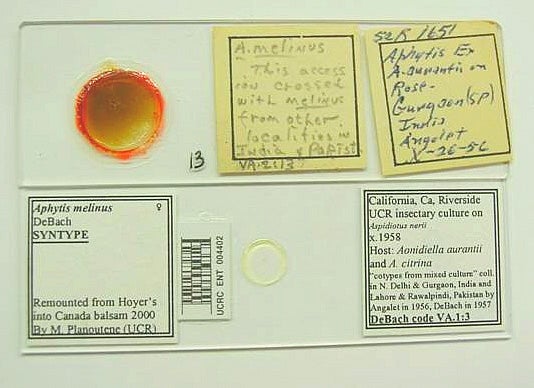
Labels from the original slide (Fig. 1) are glued to it if necessary after the specimens are removed from it by soaking it in distilled water. On top of the slide, a new label is attached which contains the bar code number(s) of the slides that contain the specimen(s) remounted from this original slide. Example: "UCRC ENT 000151-000166". The original slides will be stored separately at the end of the Aphytis collection, arranged numerically, rather than alphabetically, for easier access.
The new slide(s) is/are labeled in the following way (Fig. 1). The right label contains locality, date, collector's name, and other relevant data such as host record(s), plant record(s), P. De Bach's catalog number, any notes or other codes, etc. The left label indicates the species identification, sex indication, name of the person responsible for identification and the date (if available). In the bottom, the left label states "Remounted from Hoyer's into Canada balsam 1998 or 1999 by M. Planoutene or V. Berezovskiy (UCR)."
Finally, the bar code with the data-base number is affixed to the slide between the identification label and the coverslip, as shown in Fig. 1.
DATA-BASING
All label and other relevant data about each specimen remounted on the new slide is entered into the Biota (Windows-95) data-base. Each slide is given the unique number, which is the same number as the bar code on the slide. The bar code number is entered automatically using the Intermec bar code reader (Model 1550), connected to a Twinhead Slimnote Power Book (TE-200TZ). The number appears in the field called "Specimen code". When available, geographical coordinates, host information, etc. is added to the original label data.
STORAGE
After the remounted slides are dry enough, they are returned in the proper place in the Aphytis collection. The UCR has 6 wooden cabinets for storing the slides flat; there is space for permanent storage of all the type and some non-type material there. The rest of the slides, mainly the common species such as Aphytis melinus De Bach, A. lingnanensis Compere, etc., will be stored separately in 100-slide capacity boxes.
REFERENCES
Rosen, D. and P. DeBach. 1979. Species of Aphytis of the world (Hymenoptera: Aphelinidae). Series Entomologica Volume 17,
Platner, G. R., R. K. Velten, M. Planoutene and J.D. Pinto. 1999. Slide-mounting techniques for Trichogramma (Trichogrammatidae) and other minute parasitic Hymenoptera. Entomological News 110 (1): 56-64.

