Contents
- 1. Labeling
- 2. Pinning Insects
- 3. Point and Card Mounting Small Insects
- 4. Alcohol Preservation
- 5. Quarantine & Biological Control Vouchers
- Correct Labels for Vouchers (in 70%-95% Ethanol) of Material Reared from a Known Host in the Field
- Correct Label for Vouchers Coming from the Original Quarantine Shipment/Material
- Correct Labels for Vouchers in 70% or 95% Ethanol Coming from Quarantine or Insectary Laboratory Colonies
- Labels for Same Vouchers if Pinned, Card- or Point-Mounted
Museum Specimen Preparation Guidelines (incl. Vouchers)
by J. Heraty, D. Yanega, S. Triapitsyn
1. Labeling
Most insect field guides talk about proper pinning, but proper labeling is not emphasized as much, and can be even more important from a scientific standpoint. A specimen that is pinned poorly can often be remounted, but a specimen whose label is missing essential data is often worthless for scientific purposes. Also, the label paper itself must be of heavy stock (at least 20 pound) and acid-free, or it will deteriorate to uselessness over time.
Top priority is having the essential information, second priority is fitting that information into a small enough space (anything bigger than about 15 x 7 mm is getting too big). It can be tricky to accommodate both priorities. Sometimes, then, abbreviations are good, as long as (a) they are standard abbreviations, or unambiguous, and (b) necessary to keep the label from being too big. Sometimes you might have room for spelling out "San Bernardino National Forest", and that's fine if so, but if you have to abbreviate (and you probably should in this case), writing "SBNF" is going to confuse a lot of people. The rule of thumb I like to use is this: "If this specimen were to wind up in the hands of a foreign scientist 50 years from now, would they be able to figure out when and where it was collected?" So, you compromise, and write "S. Bernardino Nat. For." - and a complete label might look like this:
- USA: CA: S. Bernardino Co.
- 5 km E Running Spgs.
- S. Bernardino Nat. For.
- 34°13'N, 117°03'W, 2000m
- 10.iv.1998 M. Gates
There are several things to notice about this example, which should help understand why it's better than most.
- It has the country. US collectors are almost unique in the world in not being in the habit of indicating the country on their labels. You can generally squeeze "USA" in there, trust me.
- The abbreviation for the state is the standard one, that even a foreigner should be able to recognize. I have an old specimen in front of me whose label reads "Loon Lake, WN" - and I can't tell whether it's supposed to be Washington or Wisconsin. Of course, if the county name was shorter, like Inyo Co., I might use "Calif." instead, because that's even less ambiguous than "CA".
- It has the county. That "Loon Lake" label doesn't list the county, and it turns out there are 18 different Loon Lakes in Wisconsin, for instance. The collector is long dead, so we'll never know where that specimen is from.
- It gives distance relative to the nearest town that one can find on a standard map or in a gazetteer. Always try to give disance relative to the nearest well-defined (and hopefully permanent) place. In many cases, a small town is better than a big town or city, because the borders are better defined. A label that says "15 km N Los Angeles" is almost useless, for example, because no one would know when to start measuring.
- It gives elevation. When you're in the hills, a few hundred yards along the road can mean a big change in elevation, and many species of plants and animals have definite altitudinal limits; this information can be very meaningful.
- It gives the distance in kilometers, and elevation in meters. The odds are good that scientists from other countries may someday read those labels, and since only the US uses miles and feet, it's better if we use the international standard.
- Best of all, it has latitude and longitude, even if not exact to the second. In principle, if you have this information, the other data on the label is hardly even necessary. It's always good to have a DeLorme map, or even a GPS unit, when you're collecting so you can note where you are. Future scientists will love you for putting that data in there. But try to avoid using decimal lat/long figures ("116.45 W") unless you know you can print a "°" symbol, because it can greatly confuse people (if a label says "116 45" no one knows whether it means 116.45° or 116°45'). And ALWAYS make your own labels so others don't have to figure this out: I've seen labels that read 38°86' N, which is impossible.
- It gives the date in an internationally recognizable format. Most countries list day then month then year, so if you write "4-10-98" on a label, people won't be sure whether it's April 10th, or October 4th (or maybe it means "April through October"; this is why you should never use hyphens). The "lower-case Roman numeral for the month" convention is how most people solve this. It's done in lower-case so people won't confuse "I" or "II" with "1" or "11".
- It doesn't say "collected by M. Gates", which would be a waste of space. Some people might write "leg. M. Gates", which isn't so wasteful of space, but this is an outdated practice (persisting almost exclusively among butterfly collectors) which confuses many people who don't know what "leg." means (it means, essentially, "collector"). Honestly, everyone knows that a person's name on an insect locality label indicates the collector, so either variant would be redundant. That's also why it doesn't say "2000m elevation" - people will know from the units that you're talking about elevation. If material was collected by a trap, the collector name, if any, should be the person who emptied the trap.
- None of the lines on the label exceed a total of 25 characters, which is about the limit for 4-point lettering to keep within 15-18 mm length. The label is also only 5 lines long, which is about the limit for staying within 7-8 mm width.
- Though this example doesn't have it, sometimes you might have an herbivorous insect caught on a possible host plant, or a bee collecting pollen, or a wood-boring insect on a host tree, or a fungus-feeding insect on a mushroom, etc. In such cases, it's good to either include this information on one line, or - if it would make the label over 5 lines long - on a separate label below the locality label. In the present case, since I have latitude and longitude, I'd replace the "S. Bernardino Nat. For." line, and use the scientific name of the host if I knew it (e.g., "on Abies balsamea" or "on Phacelia flws."). Sometimes that sort of biological information is extremely valuable, and you don't want to omit it, though you also want to keep it down to a reasonable size or skip it altogether if it's trivial (e.g., "found on dead rabbit carcass on rocks along stream" is way too wordy, and largely trivial - "ex carcass" would be plenty).
- Finally, if you're making labels using software, choice of font is very important. Assuming you have a laser printer/inkjet that prints crisply (a VERY important assumption) the size should be 4 point lettering, with fairly close spacing between lines (but not so close that the bottom of, say, a "g" on one line will overlap the top of an "f" on the line below), and my preferred font is something like Times (or maybe New York), which has very compact letters with close spacing, and serifs on the characters. Certain sans-serif fonts (especially Arial) are bad for labels because certain characters (or combinations of them) are harder to distinguish, though it varies from font to font (e.g., at small font size, the acid test - distinguishing "rn" versus "m" - is easy in Geneva, but very hard in Arial or Helvetica). If the resolution of your printer isn't good, then you might need to use a simpler sans-serif font like Geneva.
Positioning Labels
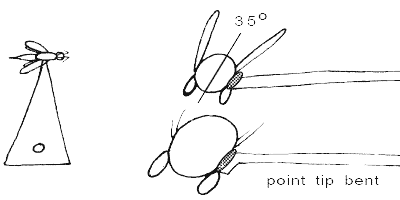
Labels should be at a maximum height of 20mm from the bottom of the pin, unless the body of the insect forces it to be lower. Labels ALWAYS have the top line oriented on the specimen's right (for regular pinned specimens) or perpendicular to a point-mounted specimen. Specimens or their points are ALWAYS oriented vertically in unit trays so the head of the insect either points to the top (pinned specimens) or to the right (point mounts). Card mounts will often have the heads pointing to the left (see below). But again, the top line of the label is always to the right. MOST IMPORTANT: The specimen plus the point/card, as a unit, should be centered relative to the label, as illustrated below. Do NOT stick the pin of a point-mounted specimen through the middle of the label, nor at the extreme edge!
2. Pinning Insects
When to pin, when NOT to pin
This is very important, and has a dual meaning: (1) There are certain types of insects which shrivel and/or discolor badly when pinned, and should therefore either be preserved in 80% ethanol indefinitely, properly dehydrated for mounting (either critical point drying or HMDS bath), or placed on microscope slides. (2) some insects are too small or too slender to be pinned, and should be point-mounted instead. Becoming acquainted, by experience, with how each type of insect preserves, will probably ultimately be necessary for most folks, but learning some rules of thumb early on will help minimize the number of disastrous pinning jobs. Improperly prepared specimens can be rendered useless for museum purposes, so you must be certain you're doing it correctly.
In the first case, which insects must go into alcohol breaks down fairly cleanly along certain lines: adults of almost any macroscopic (> 2 mm) Odonata, Hemiptera, Homoptera, Mecoptera, Hymenoptera, Diptera, Lepidoptera, and Coleoptera are perfectly suitable for pinning or pointing, with few exceptions (e.g. scale insects, aphids). When you get into the microscopic types (under 2 mm), or immature stages, then the odds that alcohol storage will be better increase dramatically. Various members of the Orthopteroid orders (including roaches, mantids, and walking sticks) lie on both sides of the fence, as do Neuroptera; many of them can be pinned, but generally speaking most of them preserve better in alcohol. If you've ever seen a camel cricket or Jerusalem cricket that's been pinned, you've seen how badly they can shrivel, even though an ordinary cricket or grasshopper might look just fine treated the same way. Likewise, a lacewing, ant lion, or dobsonfly on a pin might not look that bad at first glance, but those shriveled abdomens aren't necessary or desirable. Members of any group I did not list above (Ephemeroptera, Plecoptera, Isoptera, Psocoptera, Trichoptera, etc.) and any immature stages should generally be preserved in alcohol. The bottom line is probably this: (1) anything whose body is somewhat membranous and soft is better off in alcohol (2) aside from butterflies and moths, scaly flies like Bombyliids, and certain types of metallic beetles (e.g., Chrysina), almost nothing can be hurt by storing it in alcohol, if only temporarily. In other words, when in doubt, go with alcohol, and if it turns out to not be necessary, then you can simply remove it and pin it normally. See below for instructions regarding alcohol-stored specimens.
In the second case, judging when something needs to be pointed versus pinned is pretty straightforward, though most folks left to their own devices tend to try to pin things that are really too small for it. You should, realistically, never need to use any pin below size 1, and anything smaller than a size 0 pin literally should never be used (they are too fragile). The rule of thumb is that anything from about the size of an average ladybug (6-7 mm) on down should be point-mounted, and anything that is skinnier than 3 mm, as well, no matter how long it is.
Proper use of a pinning block, posing, etc
I consider it unfortunate that most pinning blocks aren't designed so the pin is inverted when adjusting the height of the insect: in essence, what really matters is where the TOP of the insect's body is, not the bottom, so simply pushing a pin into a pinning block can often cause problems if the insect's body is very thick. If you don't have at least 8 mm of space between the head of the pin and the top of the insect, then you have the body mounted too high, and you run the very real risk of damage to the specimen from contact with fingertips. If you have more than 11 or 12 mm of clearance, then the body is too low, and you're obviously not leaving enough space for labels. Our equipment engineer contact, John Rose, has designed a pinning block with a 10mm hole for inverted pins which solves this problem, and they can be purchased through Dave Hawks. Otherwise, practice is needed.
For very large insects, and putting aside the matter of spreading lepidopterans, some folks like to pin into styrofoam blocks, but very often the foam is too thin and the bodies are mounted too low, accordingly - pinning this way helps get the legs and other appendages nicely arranged in one plane, and prevents the abdomen drooping, but that shouldn't be at the expense of having the body at the proper height. Either use thicker foam, or use small cards (e.g. 15 x 20 mm or slightly larger) under the bodies until they're dry, in order to help get good poses without having the body at the wrong height. An advantage of pinning into thick foam instead of using cards is that one can then use brace pins to hold the appendages in place while they dry (the end results can be extraordinary, as anyone who's seen Dave Hawks' display specimens can attest). Along these lines, while having the appendages spread away from the body can improve visibility of certain structures, and make for cosmetically appealing mounts, this has to be balanced against the GREATLY increased risk of having appendages broken off if they're sticking out. A possible rule of thumb is that the tarsi and antennal tips should end up no more than 1/3 of the body width away from the body. Generally, specimens destined strictly for research are prepared with minimal fuss, and keeping the limbs and antennae in close; it saves space and minimizes breakage. Display specimens are a different matter.
As for wing-spreading with insects other than leps, it can sometimes be useful, but it should again be weighed against the increased risk, and the increase in storage space it might require. Ask yourself the following questions: (1) "Is there some potentially important structure or feature of the abdomen that I can't see if I don't spread at least one pair of wings?" (2) "Are there important features of the wings themselves that I can't see unless at least one pair is spread?". If the answer to either question is "Yes", then you should probably spread one pair of wings, generally on the left side of the body (since the pin is placed through the right side). As with leps, the back margin of the forewing should be at a right angle to the long axis of the body. Another thing to consider is that if you have multiple specimens of the same insect collected together, you can spread the wings on one, and leave the other specimens unspread; I've seen this sort of thing many times with people who collect grasshoppers, for example. Liberal use of this particular rule can lead to some interesting discoveries, too, because the abdomens of certain types of beetles and hemipterans (e.g., Buprestidae and Coreidae, respectively), sometimes have distinctive colors and markings that are worth exposing to view, in at least one specimen of a collected series. It may not be traditional for these insect groups, but sometimes new and useful taxonomic characters can be found this way, so it's worth considering.
Finally, along these lines, some insects have special requirements for making anatomical features visible that are of taxonomic importance. Megachilid bees and many Sphecoid wasps, for example, should have their mandibles spread. Chrysidid wasps need to have the underside of the abdomen exposed. Apiocerid flies and some butterflies should have the male claspers spread, and a great many insects of all types have crucial characters of the male genitalia that can be made visible simply by pulling the genital capsule out of the body either partially or entirely. All of these tricks are best done when the specimen is fresh, and it can make a difference in how one approaches preserving and mounting a given specimen. Familiarity with what is useful for each type of insect is essential if you sincerely wish to maximize the future utility of your specimens.
3. Point and Card Mounting Small Insects
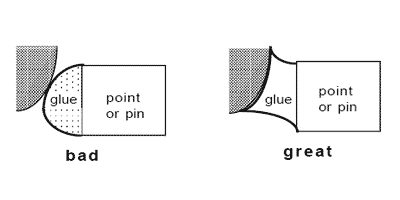
If you can't easily put a pin into an insect, then it should be point or card-mounted or, if desperate, glued to the side of the pin. With these techniques, the idea is always to use as little glue as is necessary for a secure hold, to see as much of the specimen as possible, and to make a permanent quality mount! Two types are predominant: triangle or point mounts and card mounts. Card mounts are more difficult to prepare correctly, and reduce visibility somewhat more, but offer absolute maximum protection for the specimen; this technique works best for insects which are essentially dorso-ventrally compressed, and hold their wings up above the body (e.g., very small flies and wasps, not beetles). Either points or cards must be made of acid-free paper, or they will not last over time. The suggested card stock is 3-ply strathmore board. Points should be made of acid-free 1-ply (at least 20 pound) card stock, about the thickness of an index card or only slightly thicker. The paper for points can't be too thick, since you want to be able to bend the tip so it fits the contour of the body of the insect as precisely as possible, to maximize the surface contact of glue to body (below). If you don't custom-shape the end of each point to the body of each insect, then you're not doing it right. The idea is that the point (not the glue) has to support the weight of the insect so that it will not fall off if shipped overseas and bounced around. If you can't point-mount your specimens so they can withstand a freefall drop of 4 inches (while in a unit tray) onto a book, then you haven't got the hang of it. Yes, that's a pretty severe drop, but well-mounted specimens will not come off their points.
As for glue, there are pros and cons to the various types, but for specimens smaller than 2 mm, it appears that a good shellac gel is best, followed perhaps by hide glue (commercial hide glue may need to be diluted slightly). We have limited experience with PVA, but various people swear by it - however, it cannot be obtained in glue form, but has to be made by grinding it into a powder and adding alcohol, which implies that there is some skill required in making it the right consistency. White glue is less than ideal (too watery for small specimens), though it is the easiest to obtain and use, and clear nail polish, which is fine for larger specimens, also apparently has similar drawbacks to white glue; the chemicals in both reportedly can cause some damage to the specimen over a long period of time, this becoming more of a problem as the insect involved becomes smaller, where a greater proportion of the body comes in contact with the glue, AND both apparently become insoluble, so they shouldn't be used when there's any chance the specimen needs to be removed at some later date (e.g., for dissection). Nail polish, at least, should only be used for specimens that will never need to be removed and dissected or remounted. In essence, if you're pointing ladybugs and other relatively big and sturdy insects, use whatever you like, but if you're pointing Mymarid wasps or anything else about 1 mm or smaller, or anything you know may need to be removed or slide-mounted, then you definitely want to use shellac gel - but be warned that you generally have to make it yourself (Bioquip's is unreliable), following the recipe below.
| Glue Type | Hold | Solvent | Features |
|---|---|---|---|
| Shellac gel | excellent holding power | EtOH | -slow drying -best for insects under 2 mm |
| Hide glue | good holding power | water | -variable drying (see below) -good for insects over 2 mm |
| White glue | fair holding power | water | -medium drying -poor for small specimens -acidic components? -becomes insoluble |
| Nail polish | excellent holding power | acetone | -fast drying -becomes insoluble |
| Polyvinyl Acetate (PVA, a.k.a. "Gelva") |
good holding power | ethanol | -uncertain properties -requires mortar and pestle |
Shellac Gel
- Heat 150 ml of clear or white Shellac for 20 minutes in a double boiler (beaker in water).
- After 20 minues add 10 ml of 70% EtOH and then boil for 5 minutes.
- Cool rapidly by plunging into crushed ice (precool vials in ice and pour in liquid gel).
Makes a vaseline-like glue that dries slowly. Small spot of gel is placed directly onto point or card using pin or other micro-tool, then insect is placed in position.
Hide glue
Not all hide glues are the same. Sears brand hide glue almost never dries out and thus specimens do not adhere readily when pointing. Therefore it isn't recommended for point mounts, but the slow set time may be better for positioning specimens on card mounts. Some other brands (Franklin Titebond) dry almost instantly and are better for point mounts but present the same problem for very small specimens as white glue—small drops dry too fast to adhere to the specimen. Most require some dilution with water, as they are too thick to use in raw form. Although the bottles say they are resistant to solvents, they do dissolve in water without leaving a residue on the specimen.
White Glue
Choose a water-based, non-toxic glue, such as Elmer's school glue.
Nail Polish
Clear nail polish only. May require thinning with acetone if too thick, but a small bottle can last for years.
For All specimens
The glue should also flow a little bit onto the specimen; NOT form a ball.
Positioning Point Mounts
ALWAYS use at least a number 2 pin for points. Use a pinning block to set point or card height on pin (10-13mm below head).
The optimal spot to glue the specimen is ALWAYS on the right side of the specimen just above the coxal margins (including the tops of the coxae).
The relative size of the glue drop and contact by the point attachment should be no bigger than shown. For insects like beetles which are relatively flat-bottomed, the tip of the point should not obscure the ventral midline, especially since the coxal insertions are often necessary for family ID. The glue spots should never be much larger, proportionally, than shown. Other groups of insects may require special techniques, e.g., ant workers prefer to have the point tip inserted slightly between the coxae rather than against them. If the right wing is folded down, you may have to carefully twist the point under the wing to contact the specimen properly, which may cause the specimen not to be perfectly perpendicular to the point, but as long as everything is still visible, that's okay - just try to make it look as good as possible.
Specimens should be mounted so that when finished, the specimen is oriented horizontally along the long axis, dorsal surface up, or perhaps even at a slight angle to the horizontal axis of the point tip (30° to 40°) as above. None of the head, dorsal surface of the mesosoma (thorax), abdomen or wings should ever be touched by the glue.
For very small specimens (1mm or less) use an unmodified point and a tiny drop of glue (upper illustration above). For larger specimens you should bend or even curl the tip of the point to provide a larger contact point for the glue to the specimen, as mentioned earlier. You must use your own judgment on what is good, but make sure that you do not obscure any morphological features. You should not be able to see the point or glue when you look at the specimen from its left side!
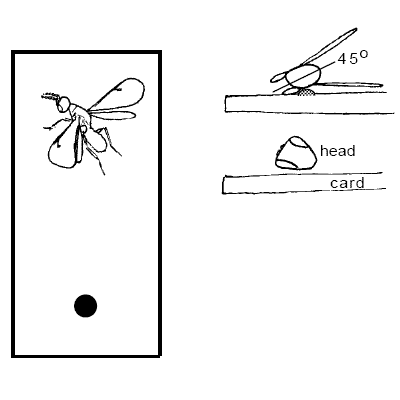
Positioning Card Mounts
ALWAYS use at least a number 3 pin for card mounts. Card mounts may be used for tiny insects or larger insects that are more or less vertically compressed and can rest on their side (e.g. Diptera, Hymenoptera, not Coleoptera), with extra care to make a good mount. Specimens are harder to mount this way because it is easier to obscure body parts, but card mounts do provide the best overall protection for specimens; they will almost never fall off.
Remember: the dorsal surface of the mesosoma and the entire frontal surface of the head must be visible. No glue on the wings if possible.
Mount (-45°) to the horizontal (long) axis of the card.The right wing is preferably flattened on the card and spread away from the specimen. The specimen is tilted slightly (45°) so that the dorsal surface is entirely visible, The specimen may be angled head higher with the abdomen flat against the card to help keep the head off the card surface. It is very important that the entire frontal surface of the head is visible. If the right wing is folded down and in the way, it may be removed and attached to the card by a drop of glue. The glue is usually applied at a thinner consistency for card mounts, to prevent premature drying. Keep a separate bottle of diluted glue for card-mounting, if necessary.
Associated host remains or prey, IF they are to be mounted together, should be glued to a point or card below the specimen and above the data label. Leaf mines or galls may be included on the mount if of reasonable size, otherwise keep separately or in a herbarium (possibly could use a plant reference code). Note that with databasing, it is possible to cross-reference specimens electronically, so it should NOT be necessary to mount different taxa on the same pin (and rarely is it desirable, accordingly)!
4. Alcohol Preservation
As mentioned above, certain types of insects like Lepidoptera and Bombyliidae should never be placed in alcohol. Hairy insects, like bees, that are kept temporarily in alcohol MUST be treated with HMDS or another special drying technique when they are removed from the alcohol, to prevent the hairs from matting. Orthopteroids and other marginally soft-bodied insects that are being pinned from alcohol should be similarly treated if they are being removed from alcohol. These sorts of insects are often better to pin immediately, if possible, rather than placing in alcohol, unless they are prone to shriveling (e.g., Neuroptera).
Storage in alcohol may be either permanent or temporary, and may or may not be needed for DNA study. For most purposes, specimens can be treated the same regardless, but in other ways it WILL make a difference. For instance, many different cap or stopper designs are not leak-proof, making them unsuitable for permanent storage. The museum will not accept vouchers unless they are in leak-proof glass or plastic vials, using either caps with plastic cone liners or rubber o-rings, or hypodermically sealed stoppers. For bulk samples, whirlpak bags are completely unsuitable, and mason jars are preferred for such large volume samples.
Specimens destined for DNA study should ideally be collected directly into 95% ethanol while still alive, into a leak-proof container, and kept as cold as possible from that moment onwards, until the DNA is extracted; the colder the better. Even a day in direct sunlight on a desk, or a few hours in a hot vehicle can cause appreciable denaturing of genetic material. Specimens preserved in 95% ethanol should be CLEARLY labeled as such. For other specimens, the concentration of ethanol should be between 70-80%, with softer-bodied specimens best kept at the low end of the scale; the use of 95% ethanol is NOT appropriate for general collecting as it causes shriveling and distortion of specimens.
Alcohol specimens should ALWAYS have one date/locality label inside the vial and another identical label taped securely on the outside: certain types of printers or papers yield labels whose writing deteriorates or separates from the paper, while labels on the outside of a vial may fall or be torn off. Redundancy is very helpful in avoiding such problems. If you are depositing a vial as a voucher, the database label you are given should go on the outside of the vial. Vials may contain multiple specimens, but insects of more than one species should never be kept together in a single vial except on a temporary basis.
5. Quarantine & Biological Control Vouchers
The museum will accept vouchers mounted on pins, cards, and points according to the general standards discussed above (all standard vouchering procedures should be adhered to: see official Museum Voucher Policy for guidelines). The labeling, however, must also follow the examples below because substantial additional information must be indicated. IMPORTANT: many of the labels below are designed for material stored in ethanol so they must be abbreviated, or broken into mutliple labels, if used for the mounted specimens (see example below and guidelines above). Because such vouchers are often submitted for identification and are small in size, like many micro-Hymenoptera, they may require special treatment prior to mounting (such as HMDS or CPD) and occasional slide-mounting. Therefore we encourage submitting such vouchers in vials (following the standards discussed above) both in 70% and 95% ethanol (if there are enough specimens); the labeling should follow the below examples.
Correct Labels for Vouchers (in 70%-95% Ethanol) of Material Reared from a Known Host in the Field
- MEXICO: Tamaulipas
- Llera de Canales
- 23°19'N, 99°01'W
- 20.ix.1998, S.N. Myartseva
- Ex Microcentrum rhombifolium
- (Saussure) eggs on lemon leaf
- USA: California, Napa Co.
- Oakville, Mondavi Vineyard
- Ex parasitized nymphs of grape whitefly,
- Trialeurodes vittata (Quaintance),
- on Vitis leaves, coll. 26.ix.2001,
- em. 1-8.x.2001, L.G. Varela
Correct Label for Vouchers Coming from the Original Quarantine Shipment/Material
- USA, Florida, Jefferson Co.
- 30°32'N, 83'52'W, 70m
- Monticello, University of Florida
- North Florida Research &
- Education Center, 30.vii.1997
- S.V. Triapitsyn. Ex parasitized
- egg mass of Homalodisca coagulata
- (Say) on Lagerstroemia indica leaf
- Emerged in UCR quarantine
- 2.viii.1997, coll. E. White
- S&R 97-18-02
Correct Labels for Vouchers in 70% or 95% Ethanol Coming from Quarantine or Insectary Laboratory Colonies
- USA: California, Riverside Co.
- Riverside, UCR campus
- F2, quarantine lab. culture
- 3.ix.2001, D.J.W. Morgan
- Host: Homalodisca coagulata
- (Say) eggs on citrus leaves
- Originally from: S & R 97-18-02
- USA, Florida, Jefferson Co.
- Monticello, University of Florida
- North Florida Research &
- Education Center, 30.vii.1997
- S.V. Triapitsyn, ex parasitized
- egg mass of Homalodisca coagulata
- (Say) on Lagerstroemia indica leaf
- Emerged in UCR quarantine
- 2.viii.1997, coll. E. White
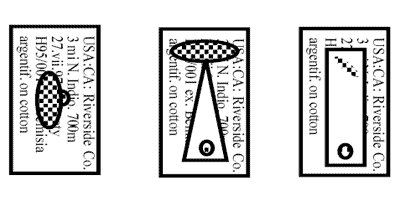
Labels for Same Vouchers if Pinned or Card or Point Mounted
- USA: CA: Riverside Co.
- UCRiverside q-ne lab. culture
- 3.ix.2001, D.J.W. Morgan
- ex Homalodisca coagulata
- eggs on citrus leaves, F2
- Orig. from: S&R 97-18-02
- USA, Florida, Jefferson Co.
- Monticello, UFNFREC
- 30.vii.1997, S.V. Triapitsyn
- ex egg mass of
- Homalodisca coagulata on
- Lagerstroemia indica leaf.
- Em. in UCR q-ne, 2.viii.1997